Editor’s Note (7/9/24): Lisa Pisano passed away on July 7. The heart pump that was implanted in Pisano failed to supply enough blood to her transplanted pig kidney, and the organ was removed on May 29. Pisano was put back on dialysis, and she later entered hospice care. Her doctors hope to learn a lot from the explanted organ in their quest to make xenotransplantation a reality.
People with heart failure may be eligible to receive a heart transplant; people with kidney failure may be lucky enough to receive a kidney transplant. But for many people with both heart and kidney disease—who may be ineligible to be listed for a transplant or cannot receive both organs in time—options are extremely limited.
Scientists at NYU Langone Health in New York City, however, have now completed a first-of-its-kind procedure involving surgery to install a left ventricular assist device (LVAD), or mechanical heart pump, followed by transplantation of a genetically modified pig kidney. It is the first time someone with an LVAD has received a transplant of any kind (except for a heart transplant to replace the device) and only the second time a genetically modified pig kidney has been transplanted into a living person.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The achievement creates a potential avenue for treating people with both heart and kidney disease. “Our success in this one patient is going to open up a lot of possibilities for the thousands of patients out there who have some combination of problems with the heart and the kidney,” says Nader Moazami, chief of the division of heart and lung transplantation and mechanical circulatory support at the NYU Grossman School of Medicine, who performed the LVAD surgery.
There is enormous demand for kidney and other transplants but not nearly enough donor organs available. In the past few years, however, scientists have made significant progress in transplanting organs from nonhuman species to humans, known as xenotransplantation.
In late March surgeons at Massachusetts General Hospital announced they had completed the first genetically modified pig kidney transplant in a living person: a 62-year-old man with end-stage kidney disease named Richard Slayman. After an early episode of immune rejection, which was successfully treated, Slayman was discharged to live at home and is doing well, according to his transplant team.
The recipient of the recent LVAD surgery and kidney xenotransplant was Lisa Pisano, a 54-year-old grandmother in New Jersey who had heart failure and end-stage kidney disease and was on dialysis. Pisano was ineligible to receive a human heart and kidney transplant because she had many contraindications that would have made both finding an organ and surviving the procedure unlikely, and her life expectancy was very limited. Her experimental surgery was performed under the U.S. Food and Drug Administration’s Expanded Access program, sometimes referred to as “compassionate use”—a pathway for people who have a serious disease with few treatment options.
First, a team led by Moazami implanted an LVAD in Pisano on April 4. Most people who are on dialysis are ineligible to receive an LVAD because there is a very high mortality rate in such individuals. That’s where the xenotransplanted kidney came in.
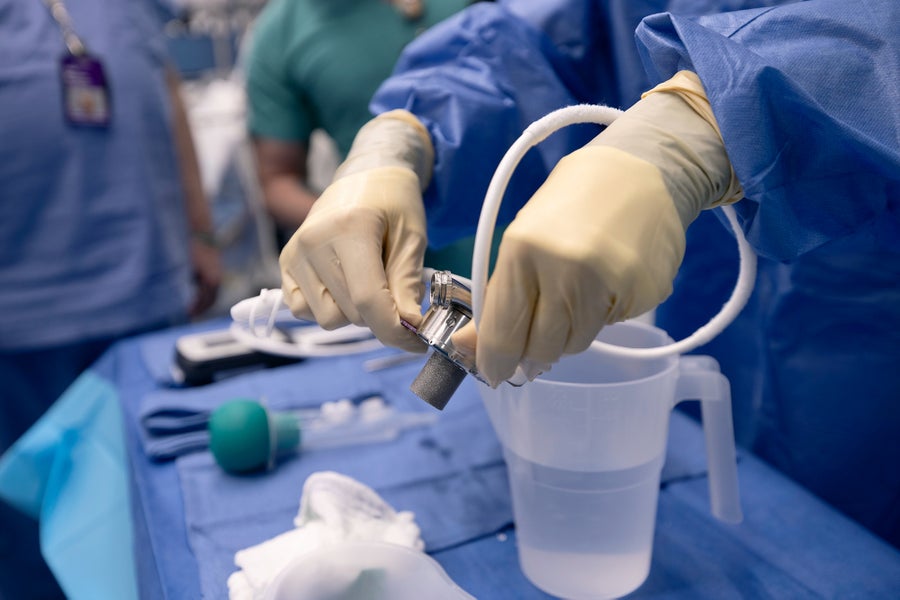
The left ventricular assist device, or LVAD, is prepared for implantation into Lisa Pisano on April 4, 2024.
Haley Ricciardi/NYU Langone Health
On April 12 a team led by Robert Montgomery, chair of the department of surgery and director of the NYU Langone Transplant Institute, transplanted the kidney with an embedded thymus gland of a pig into Pisano. The pig had been genetically edited to remove a gene that results in the production of a sugar called alpha-gal—which can trigger an immune response in the human body that could cause it to reject a pig organ. The thymus gland was included in the hope that it might ultimately reduce the amount of immunosuppressive medication needed after transplant by training the immune system not to attack the foreign kidney.
The team had to figure out the right flow rate for the heart pump to allow the transplanted kidney to function effectively. Yet as soon as they did, the organ started producing urine and creatinine, a key waste product that kidneys are supposed to remove. “There were things we had to learn because it hadn’t been done before,” Montgomery says.
There were some important differences between the NYU Langone xenotransplant and the earlier one at Mass General. “There are basically two potential ways of thinking of how xenotransplantation may help patients,” says Leonardo Riella, chair of transplantation and medical director for kidney transplantation at Mass General, who helped lead the team that performed the earlier pig kidney transplant.“On one side, we have the kidney transplant candidates that are on the waiting list, who unfortunately don’t have a donor—but they have been approved to receive a human kidney transplant,” he says. That was the case with his team’s xenotransplant recipient, Slayman. The other way xenotransplantation could help, Riella says, is with “a patient who’s not a candidate for a human organ transplantation but [who is offered] the xenotransplant as a potential last resort,” as was the case with Pisano.
Pisano was likely much sicker than Slayman, Riella says. Unlike Slayman, she was not eligible for a human organ transplant, and she also had both heart and kidney failure, whereas he had kidney failure alone. “There are values in both [types of surgery],” Riella says. Yet “doing it with a patient who’s not a [human organ] transplant candidate usually carries higher risk.”
Additionally, Pisano received an organ from a pig that had had only one genetic modification, whereas Slayman received one from an animal with 69 genetic edits. It remains to be seen whether such additional edits are necessary for the long-term survival of such xenotransplanted organs in people.
Pisano has so far shown no signs of acute immune rejection. The critical point for that may come around a month after surgery, however, Montgomery says. For now, he and his colleagues are continuing to monitor her closely.
“I do think it’s incredibly exciting we now have a second genetically edited pig kidney that's been put into a living person,” says Jayme Locke, director of the division of transplantation and chair of transplantation surgeryat the University of Alabama at Birmingham, whose team has performed previous pig kidney transplants in humans who have had brain death. “Like with any other transplant, in the early days, we want to make sure that we have control of the immune system and that there aren’t early rejections,” she says. “If this works, that’s incredibly positive.”
The two recent kidney xenotransplants will provide scientists with valuable knowledge about the procedure’s safety and effectiveness. But both the NYU Langone and Mass General teams note that clinical trials in a larger group of people are an important next step before xenotransplants can become an approved treatment and help to address the immense need for organs.
As for Pisano, Montgomery says that she knew the risks of the procedure going in, but she felt it was worth it for the chance of extending her life. “She knew she was going to die and that she didn’t have much time,” he says. “Right from the beginning, she said, ‘I want to spend time with my grandkids and have some more time.’”