Three scientists involved in developing the blockbuster anti-obesity drugs that are currently changing the health-care landscape are among the winners of this year’s prestigious Lasker Awards. The prizes, which honour important advances in medical research, are often considered an indicator of whether a specific advance or scientist will win a Nobel Prize — and some are speculating that this could soon be the case for the weight-loss treatments.
Joel Habener, Svetlana Mojsov and Lotte Bjerre Knudsen each contributed to the creation of the popular anti-obesity drugs, which mimic a hormone called glucagon-like peptide 1 (GLP-1), involved in lowering blood-sugar levels and controlling appetite. The trio, recognized with a Lasker in the clinical-research category, will share a US$250,000 prize.
Biomedical scientists are enthusiastic about the increasing recognition of GLP-1 research, which was initially aimed at treating diabetes. “I’ve been working on this for 30 years, and for a long time nobody cared,” says Randy Seeley, an obesity specialist at the University of Michigan in Ann Arbor. “Over the last several years, the situation has changed so much. We now have therapies that are actually helping people.”
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Other recipients of this year’s Lasker Awards include Zhijian ‘James’ Chen at UT Southwestern Medical Center in Dallas, Texas, who was honoured in the basic-research category for discovering how DNA triggers immune and inflammatory responses. In the public-service category, Salim Abdool Karim and Quarraisha Abdool Karim, both at the Centre for AIDS Programme of Research in South Africa, in Durban, were recognized for developing life-saving approaches to prevent and treat HIV infections.
Inside the science
Habener, an endocrinologist at Massachusetts General Hospital in Boston, was a leader in discovering the GLP-1 hormone in the 1980s. He was interested in understanding the hormones involved in type 2 diabetes, a condition characterized by high blood-sugar levels, in which the body either doesn’t produce enough insulin or has trouble using it to absorb sugar from the blood.
Habener zeroed in on glucagon, a hormone that increases blood-sugar levels. After cloning the gene for glucagon, he discovered that the gene also encoded a related hormone — later named GLP-1 — that stimulates the pancreas to produce insulin.
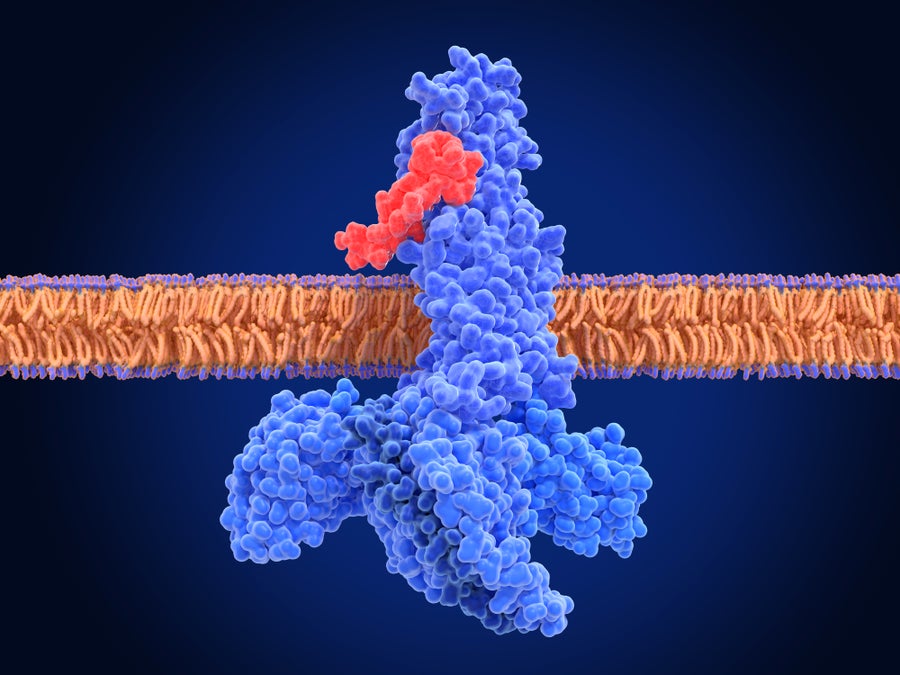
Illustration of a glucagon-like peptide-1 (GLP-1) receptor (blue) binding to a semaglutide molecule (red), forming an activated complex. Semaglutide is a GLP-1 receptor agonist, a type of drug that mimics the function of natural GLP-1 hormones.
Juan Gaertner/Science Photo Library/Getty Images
“This was interesting because, rather than having to give injections of insulin to people with diabetes to control blood sugar, giving GLP-1 would theoretically prompt the body to make its own insulin,” Habener says.
Around that time, Mojsov, a biochemist who directed a facility producing synthetic proteins at Massachusetts General Hospital, identified the sequence of amino acids making up the biologically active form of GLP-1. Eventually, she would demonstrate that this active form could stimulate insulin release from a rat pancreas — a necessary step on the path to a human treatment.
Now at Rockefeller University in New York City, Mojsov spoke out last year about the lack of recognition for her contribution to the field. Since then, she has received awards such as the VinFuture Prize. “I’m happy that I’m getting awards, but what makes me even happier is that people are actually reading my work,” she says.
After the initial discoveries about GLP-1, researchers realized that there was a significant obstacle to its therapeutic use: the hormone was rapidly metabolized, lasting only a few minutes in the blood. That’s where the work of Knudsen, a scientist at pharmaceutical firm Novo Nordisk, in Copenhagen, came in. She and her team realized that regular GLP-1 was not going to work as a medicine, Knudsen says. Instead, the researchers came up with a way to modify GLP-1 by attaching a fatty acid to it — an alteration that allowed the molecule to remain active in the body for an extended period before degrading.
The work resulted in liraglutide, the first long-lasting GLP-1-based drug, approved by the US Food and Drug Administration in 2010 for type 2 diabetes. In the meantime, researchers were already exploring the drugs’ weight-loss potential, and in 2014, liraglutide became the first molecule in its class to be approved for treating obesity. Today, newer variants, including semaglutide and tirzepatide, sold as Wegovy and Zepbound, are important obesity treatments.
“I really hope to inspire young people so that they can see that you can do great science also in the pharmaceutical industry,” Knudsen says.
Nobel ahead?
GLP-1-based drugs don’t just treat obesity and diabetes. Studies have shown they can help with cardiovascular disease, sleep apnea and kidney disease, among other conditions. These benefits are thought to arise from the drugs’ effects on the brain, as well as their anti-inflammatory potential.
Owing to the shake-up these drugs are causing in health care, some think they might soon win science’s top prize — the Nobel. Winning a Lasker often precedes winning a Nobel prize: since 1945, 95 Lasker laureates have also received that top honour. “This raises the spectre that the Nobel committee will take [GLP-1 research] seriously,” Seeley says. The Nobel prizes will be announced next month.Each prize in a science discipline is limited to no more than three winners, and the challenge will be to select the most deserving recipients. Several other scientists involved in the research behind GLP-1-based drugs have been recognized by other awards, including Jens Juul Holst at the University of Copenhagen, Daniel Drucker at the University of Toronto in Canada, and Richard DiMarchi at Indiana University in Bloomington.“It’s 10,000 ants that move the anthill, and we’re trying to pick out the three ants that made the most difference,” Seeley says. “You could come up with a dozen names of people, at least, who have made seminal contributions to the field.”
This article is reproduced with permission and was first published on September 19, 2024.
