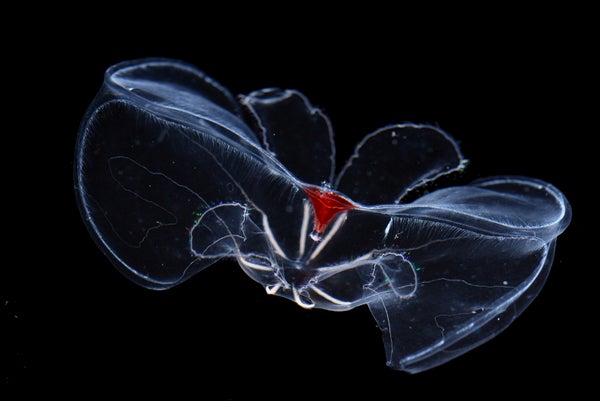
In the ocean’s depths, seawater’s punishing weight would crush most surface-dwelling species to a pulp. So how do ctenophores—squishy, see-through creatures with bodies the consistency of Jell-O—thrive kilometers deep?
Research published in Science explains how deep-sea ctenophores keep it together under extreme pressure and why they “melt” like the Wicked Witch of the West when brought to the surface. “For some deep-sea ctenophores, their cell membranes are literally held together by pressure,” explains the study’s lead author, Jacob R. Winnikoff, a deep-sea biochemist at Harvard University.
Ctenophores, also called comb jellies, are ghostly-looking bags of goo whose crystalline combs—structures they use like tiny oars to move through water—refract light into rainbows. Despite their ethereal looks, they’re voracious predators found slurping up plankton, crustaceans and small fish from pole to pole of our watery globe. And despite their name, they’re not closely related to jellyfish.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
To suss out what makes deep-water ctenophores so graceful under pressure but oozy at the surface, researchers around the world collected comb jelly species that live at a range of depths. Scuba divers scooped shallow-water ctenophores from the ocean off Hawaii’s Big Island and in the Arctic, for instance, and remotely operated vehicles gently vacuumed up those creatures’ cousins at depths of up to four kilometers below the waves off the coast of California.

Comb jellies have varying levels of the phospholipid PPE based on their native depths. This shallow-water comb jelly species would have lower levels than its deep-sea bretheren.
Jacob Winnikoff/Harvard University
Comparison of the animals’ body tissues revealed that the deeper a comb jelly lives, the higher its level of PPE, short for plasmenyl phosphatidylethanolamine—a variety of cone-shaped phospholipid, which is a fatty molecule found in cell membranes.
At high pressures all molecules are slightly “squeezed” out of shape, Winnikoff explains—and because lipids are particularly squishy, cone-shaped lipid molecules warp into cylinders in the ocean’s depths. Usually combinations of cone- and cylinder-shaped lipids balance a cell membrane’s stability and flexibility. Without enough cones the cylinders lock together like bricks, and the business of the cell breaks down, he says. Proteins, the “machines” of the cell, don’t have the wiggle room they need to move and operate. Signals can’t enter or exit, and the cell is functionally paralyzed.
By using a particle accelerator to map out PPE’s structure, Winnikoff and his team discovered that the molecule’s cone flares more dramatically than that of any other phospholipid ever documented. PPE’s shape is exaggerated enough, the researchers found via modeling, to remain a cone even under compression.
But there’s a downside: comb jellies adapted to life in the deep needthat high pressure to keep their membranes intact. If it’s reduced, PPE’s conical shape expands; this change causes cell membranes to ripple, crack and ultimately curl up into nanoscopic “macaroni” shapes, the researchers found.
On a whim, the scientists next used genetic engineering to increase PPE levels in Escherichia coli bacteria, replacing about a quarter of the bacteria’s phospholipids with PPE. They found that although E. coli’s growth typically slows under pressure, the new, higher-PPE strain “performed exactly the same” at surface pressure and a simulated depth of five kilometers, Winnikoff says.
The team’s findings are “fantastic” and “answer a very, very long-standing question about ctenophores,” says Cornelia Jaspers, an ecologist studying these animals at the Technical University of Denmark. Sanna Majaneva, a marine ecologist at the Norwegian aquatic research institute Akvaplan-niva, says it’s gratifying to finally know why so many of her specimens have “dissolved,” “crumbled” and “shivered” apart before her eyes since she began working with ctenophores more than a decade ago.
This work could aid research on landlubbers, too: PPEs are part of the human nervous system, and their loss is associated with conditions such as Alzheimer’s disease. Pinning down the extent of PPE’s curve, as done for the first time in this study, and knowing how to manipulate levels of the molecule could suggest new avenues to explore in the search for neurological treatments, says co-author Itay Budin, a biophysicist at the University of California, San Diego. “It’s not just relevant to the deep sea,” Budin says.