If all 62 million metric tons of electronic waste the world produces in a year were loaded into garbage trucks, they’d encircle the planet bumper to bumper, according to a recent United Nations report. And hidden in that monstrous traffic jam would be startling amounts of precious metals, including gold—crucial in electronics because it conducts electricity, stretches into wires and won’t easily corrode. Modern iPhones have gold in their cameras, circuit boards and USB-C connectors. Pound for pound, there is more of the substance in cell phones than in ore from a typical gold mine.
But wringing precious metals from trashed electronics is a harsh business. Using energy-intensive smelters, recycling facilities process e-waste with punishing heat. Or caustic agents are used to break down bulk electronics into liquids full of metal ions. This approach then requires complex electrochemical processes and toxic treatments to extract valuable elements in their metallic forms. The search for environmentally friendlier methods that skip those additional steps has led materials scientists down some unusual alleyways. For instance, an aerogel made from whey protein—a cheese by-product—can capture gold ions from computer motherboards bathed in acid.
An experimental sponge, described in the Proceedings of the National Academy of Sciences USA, combines graphene oxide (a thin sheet of carbon and oxygen molecules) with chitosan, a sugar derived from shrimp shells, to strain out gold. Because chitosan spontaneously attaches to the carbon sheet, the sponge essentially builds itself.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
In their initial experiments, the study authors used the sponge to filter water that contained gold ions. The pale yellow liquid became clear as gold particles piled up in the material’s molecular mesh. Once there, the ions reacted with the chitosan, which, as a natural reducing agent, helped to transform the gold back into its metallic form.
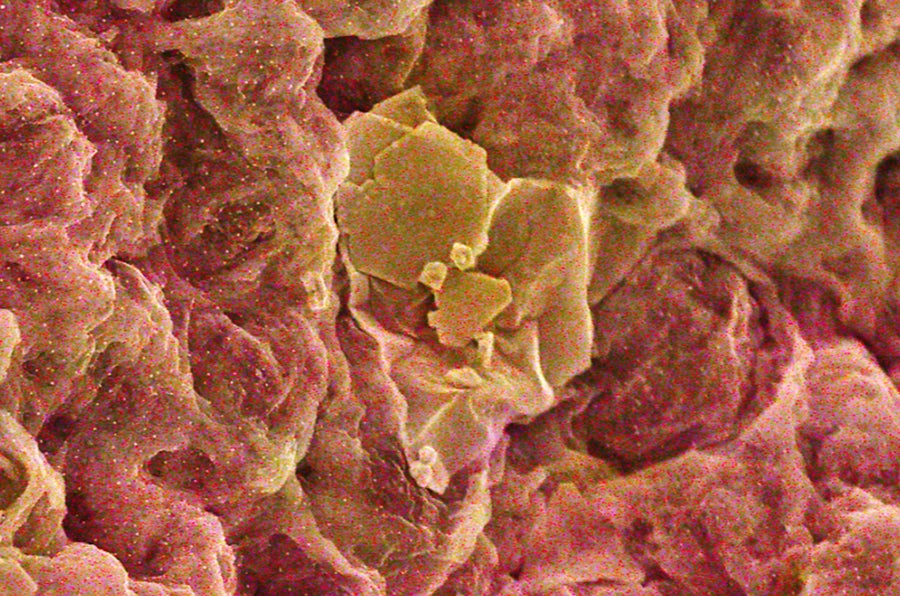
Gold (yellow) nestles in a graphene-chitosan sponge.
Kou Yang
The researchers also tested the sponge on partially processed e-waste; when they increased the acidity of the liquid to a pH of 3, the sponge’s chitosan captured the remaining gold while ignoring other metals. Although it requires an acidic environment to work, the sponge could eliminate the need for further processing, which can rely on poisonous substances such as cyanide to obtain metallic gold from liquids. “Our method allows for efficient recovery of gold directly from the waste mixture,” say study co-authors Daria Andreeva-Baeumler and Konstantin Novoselov, materials scientists at the National University of Singapore.
The new material is one of the most potent gold adsorbers ever created. (“Adsorption” is similar to the more familiar “absorption,” but adsorbed things accumulate on surfaces, whereas absorbed things are internalized. It’s the difference between a mustard glob on your chin and the hot dog you just ate.) The sponge collected up to 99.5 percent of the gold by weight from liquids with gold concentrations as low as three parts per million.
“To the best of my knowledge, this is a record-high value,” says ETH Zürich physicist Raffaele Mezzenga, an author of the whey protein aerogel study, who wasn’t involved with the graphene-chitosan research. He notes that as efficient as the sponge is, the components needed to make it aren’t cheap, and he questions whether it would be a workable option “under real operating conditions.” Adapting the technique for industrial-scale use, Novoselov and Andreeva-Baeumler say, “is indeed the next step in our research.”
