Sonja Salmon is a big fan of cellulose, and that’s why she wants to destroy it. “I love cellulose,” she says. “I’m ripping cellulose apart because I love it.”
She’s also pulling it apart because the polymer, which is found naturally in wood and cotton, accounts for one-quarter of all the fibres used in textile manufacturing. That means any effort to recycle clothing and fabric to keep them part of the circular economy for as long as possible has to include ways to deal with all that cellulose.
Salmon, a polymer scientist at Wilson College of Textiles, North Carolina State University in Raleigh, is working on breaking down the cellulose from discarded textiles and reusing it. Many clothing fabrics are a blend of half polyester and half cotton—individual fibres of cotton and polyester are twisted tightly around one another, creating a yarn that is then woven or knitted into a garment. Taking that structure apart mechanically is challenging, so instead Salmon treats it with cellulases, a group of enzymes that break up the cellulose. “We can chew it up into small enough molecules and fragments that it will actually fall out of the rest of the fabric structure,” Salmon says.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Her focus is on characterizing the material that comes out of the breakdown process and working out what it might best be used for. For example, the enzymes break down the cellulose into glucose, which could be used as a feedstock for making biofuel. They also leave behind tiny chunks of cotton fibre that could provide lightweight reinforcement for concrete. “Even though the cotton fibre will no longer be long enough to directly spin it back into a yarn, we think the material has value,” Salmon says.
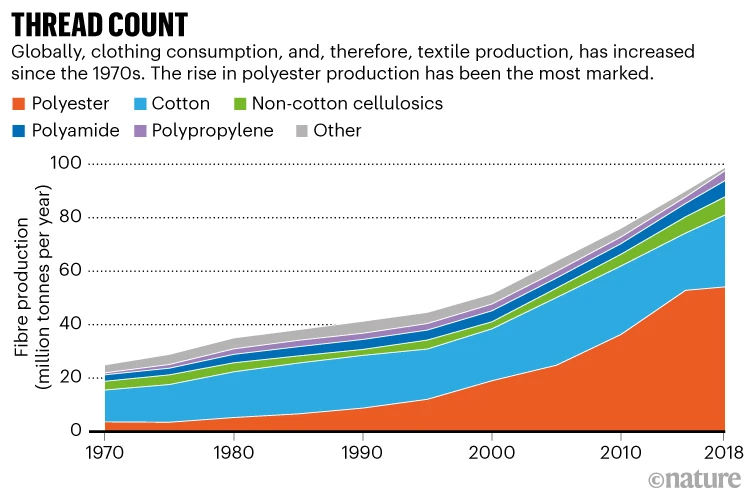
This way of thinking is a big change from how old clothing and textiles, such as upholstery fabrics and carpeting, are currently handled. Globally, only 13% of the material that goes into making clothing is recycled, according to the Ellen MacArthur Foundation, an organization in Cowes, UK, that promotes the circular economy. Most textile waste—an estimated 92 million tonnes from the fashion industry alone—produced each year winds up buried or incinerated. “We throw stuff away into landfill and we’re treating it like garbage,” Salmon says. “We’re not looking upon it as something that is actually a raw material that could be reused.” The US Environmental Protection Agency estimated that, in the United States in 2018, the average person threw away 47 kilograms of textiles. About three-quarters of that— 36 kg—is clothing and footwear, while the rest is mostly towels, bedding, furniture fabrics and carpets. Meanwhile, resources are expended to create virgin material (see ‘Thread count’)—water and land to grow more cotton, and petroleum to make more polyesters (see ‘Recovering polyester’).
To counter all that waste, researchers and start-up companies are developing methods to recover and reuse the material. Similar to Salmon, much of their focus is on chemical recycling, in which the material is broken down into its building blocks and used to create new materials, including fibres that can be woven into new clothes. The challenges lie in developing the processes for such treatment. They have to be practical, but they also have to be at least as cost-effective as simply making new fibres.
Source: K. Niinimäki et al. Nature Rev. Earth Environ. 1, 189–200 (2020).
Spinning new threads
In addition to the natural cellulose fibres from cotton, some textiles include human-made cellulosic fibres. These fibres are derived from wood-pulp cellulose and can be used to make materials such as viscose (rayon) and a similar material called lyocell. Cellulosic fibres make up around 6% of all textile fibres produced, according to the Textile Exchange in Lamesa, Texas—a non-profit organization that promotes environmentally friendly materials.
A variation on the lyocell-manufacturing process is being applied to the textile-waste problem by Evrnu, a start-up in Seattle, Washington. One major change the company has made to the process is it uses discarded textiles, instead of wood, as the source of its cellulose. Its also tweaked the process to produce a fibre that the firm’s co-founder and president Christopher Stanev says is superior to both other cellulosics and to cotton, and that can be recycled more times. “We can make much stronger fibre using cotton than the one coming from wood pulp,” says Stanev, a textile engineer.
In the same way as the standard lyocell process, the raw material is treated with N-methylmorpholine N-oxide (NMMO), an organic compound that dissolves cellulose. This produces a thick pulp that is then filtered. At this point, the conventional process would involve the cellulose being extruded through a device called a spinneret—first into air, and then into a coagulation bath of mostly water in which the material solidifies into fibre. Evrnu, however, turns the cellulose molecules into liquid crystals before they are extruded, allowing them to align with each other and produce a more crystalline fibre structure.
Recovering Polyester
Cellulose isn’t the only polymer researchers want to reuse—they also have polyester in their sights
Polyester is a generic term for a range of polymers derived from petroleum, but it mainly refers to polyethylene terephthalate (PET). Globally, PET polyester makes up around half of all fibre in all textiles. Cotton comprises another one-quarter and the rest consists of other plant-based fibres, such as linen and hemp; animal products, such as wool and alpaca; other synthetics, including acrylic and nylon; and human-made cellulosic fibres.
Like cotton, PET polyester can be spun into new fibres, but the re-spun fibres become shorter and weaker with repeated cycles. Unlike cotton, however, the polymer could be broken down into the simpler molecules that make it up and those monomers could then be reconstituted into new polymers. Starting with waste PET, Sonja Salmon, a polymer scientist at North Carolina State University in Raleigh, says, it’s possible to create what is essentially a virgin material—one that is indistinguishable from PET made from petroleum. PET is extremely stable, however, so reducing it to monomers is difficult.
Some scientists are developing enzymes that might be able to tackle these molecules. In 2016, a team discovered a bacterium that could break down PET (S. Yoshida et al. Science 351, 1196–1199; 2016), and scientists have since developed other enzymes to degrade it (J. Egan & S. Salmon SN Appl. Sci. 4, 22; 2022). Christopher Stanev, co-founder of Evrnu in Seattle, Washington, says alongside its main focus of breaking down cellulose, the start-up is also working on processes to break down PET and polyurethane, and to separate polyester–cotton blends.
“By doing that and having quite a crystalline organization, you can increase the strength and you can also engineer the performance of this fibre,” Stanev says. He says the fibre is about 20% stronger than standard lyocell, which itself is stronger than cotton.
That quality translates into a longer lifetime for a fabric made from the fibre, as well as a fibre that can be reconstituted several times. Every time the molecules are run through the recycling process, they become shorter and thinner. But because they start out stronger, Stanev says, the same material should be able to be reconstituted at least five times before it becomes weaker than virgin cotton fibre; some tests in the company’s laboratory show that the material can be recycled up to ten times. That’s more than is possible for paper, which can be recycled 5–7 times before the fibres become too short to make a viable new product.
Evrnu is running a pilot project at partner companies in Germany and elsewhere in the United States to show that its process can produce fabric. It hopes that a larger textile company will then want to license the technology. For now, it is using NMMO because the compound is readily available, but Stanev hopes to eventually switch to an ionic liquid—a salt that is liquid below 100 °C—which is more chemically stable than NMMO and more tolerant of contaminants. The firm has not yet optimized any such liquids for the production process.
A Finnish company, however, is working with an ionic liquid developed by one of its founders, physical chemist Herbert Sixta at Aalto University in Espoo, Finland. The liquid used by Ioncell—the name of both the company and the process—is a superbase, a highly alkaline substance that breaks the hydrogen bonds in the cellulose molecules. In the same way as when using NMMO, that process creates a pulp that can be fed through a spinneret to make a new cellulose fibre. NMMO tends to be unstable and requires the addition of buffer solutions, but the ionic liquid does not. Sixta says his ionic liquid is also completely recyclable, making the process environmentally friendly as well as producing fibres with better mechanical properties than cotton.
The Ioncell process can use wood pulp, which Sixta says counts as part of a circular economy because the raw material comes from Finland’s sustainable forests—these are managed in such a way that growth outpaces the amount removed. “Our university has a large group in textile design, so we can treat wood, produce pulp, convert it to fibres, convert it to yarns, convert it to fabrics, design clothing, and show the clothing in fashion shows,” Sixta says. The process can also accept textile waste, turning old clothing into new garments. Ioncell has built a pilot plant, with the goal of evaluating how well its process works in the real world in about two years.
A matter of cost
Although technical challenges abound, the main barrier to widespread textile recycling might be economic, says materials engineer Youjiang Wang at the Georgia Institute of Technology in Atlanta. “Most of the materials are not that valuable,” Wang says. It’s so cheap to produce polyester, cotton and other fabrics that there’s little profit margin unless the recycling processes are very inexpensive.
There’s also a lack of infrastructure for collecting and sorting used textiles, beyond a few private clothing-donation groups. And the complex mixture of materials in a piece of clothing—not just different natural and synthetic fibres, but also dyes and chemical coatings, buttons and zips, and any non-woven additions such as leather or latex—must be separated for individual components to be processed.
Policymakers should consider recycling that turns used clothing not into new clothes but into other useful—if lower value—products, Wang argues. Fibres might be shredded for use as soil stabilizers, for instance, or cellulose broken down into glucose that can be turned into fuel. Even burning polyester for energy is preferable to pulling more petroleum out of the ground to produce power. “That doesn’t sound very high tech, but overall, you do get considerable benefit from that,” Wang says.
The circular economy should be viewed as a way to reduce as much as possible the creation of virgin material when other products can be reused, Wang says. “If you really want to make recycling better for the environment, not just for the sake of publicity, then we need to develop more technologies so that you can use as much of what you collect as possible,” he says. “That would make the overall circle more circular.”
This article is part of Nature Outlook: Circular Economy, an editorially independent supplement produced with financial support from Google. About this content.
