Every day people with common mental health difficulties receive prescriptions for therapies that will not help them. Finding treatments that work for these patients entails an arduous process of trial and error. Each failed therapy risks leaving a patient despondent about whether anything will ever help.
Depression illustrates poignantly what can go wrong. By most measures, half to two thirds of patients diagnosed with depression will fail to benefit from any particular treatment. Research protocols for depression consist of clinical trials that typically evaluate the general effectiveness of a drug or behavioral therapy based on the average benefit for a patient. They overlook, however, the wide range of individual patient outcomes, ranging from full recovery to no benefit at all. The largest and longest evaluation of drug treatment for depression, a National Institutes of Health study of thousands of patients at multiple U.S. health care institutions called STAR*D, illustrates what can happen. Every patient in the study received an initial drug, and about a third showed major improvements. Only about a quarter of those who failed to respond to the first drug benefited from the second. After a third and fourth prescription for other drugs, 70 percent of patients demonstrated substantial progress. But most had to experience one or more treatment failures before finding a drug that worked.
Failed treatments not only prolong distress, they also discourage patients from seeking help. Participants in STAR*D knew they had possible access to other treatments in the next phase of the study, but even so many gave up. A substantial number of patients dropped out of the study after an initial failed pharmaceutical treatment, about 30 percent after a second therapy and about 42 percent following a third. (Behavioral treatment of depression using the form of talk therapy known as cognitive-behavioral therapy, or CBT, also yields a strong benefit for about half of patients.)
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Explanations for the difficulties psychiatrists face relate to the imprecisions and economic imperatives of drug development. Two people diagnosed with the same mental health disorder can respond in wholly different ways to the same drug treatment because of the current inability to assess who will respond to which treatment. Yet pharmaceutical companies typically aim for the largest possible market rather than tailoring treatment to smaller patient groups that exhibit a specific form of depression or another psychiatric disorder. Drug developers also lack the tools to implement a more precise approach. Diagnostic techniques to predict whether a person will profit from a given treatment are not part of routine medical practice.
In recent years various brain-imaging techniques, combined with sophisticated algorithms that analyze neural activity, have started to reveal brain differences among people that predict whether a given drug or talk therapy will lift a patient out of a depression or relieve severe social anxiety. Early versions of these diagnostic techniques have also shown promise in determining whether an alcoholic might relapse—and they have even begun to identify whether a student will face educational difficulties in reading and mathematics.
Brain scans to tailor treatments embody a new form of personalized medicine, an approach that often relies on customizing therapies based on an individual's genetics. Undoubtedly, genes can predispose a person to mental illness. For any one individual, though, only a weak relation exists between a given gene and common psychiatric disorders. Experience also plays a pivotal role in determining which genes become activated in the brain. Although imaging has many limitations, it approximates what is happening in the brain through the combination of genes and experience. At the moment, it can forecast the prospects for a treatment with greater precision than genetics alone. As these techniques are refined, however, the melding of brain and genetic measures may one day offer still more accurate predictions.
Will it Work?
A study that my group at the Massachusetts Institute of Technology performed in collaboration with clinician-scientists at Boston University and Massachusetts General Hospital demonstrates the prospects for predicting whether a treatment might work. Together we studied how patients with social anxiety disorder responded to CBT. Social anxiety, characterized by an intense fear of interacting with others, remains one of the most common psychiatric conditions in the U.S. Its severe form is often so disabling that the affected person cannot hold a job. In our study, all patients received behavioral therapy. We wanted to find out whether brain measurements taken before treatment could predict who would benefit substantially from CBT.
Patients viewed faces with either neutral or negative (angry) emotional expressions while we recorded responses using functional magnetic resonance imaging (fMRI), a type of scan that measures changes in brain blood flow. We also asked a series of questions to quantify the severity of their anxieties. Patients with greater responses to angry faces in regions at the back of the brain, which processes faces and other visual objects, were more likely to benefit from CBT. Using such a brain measure more than tripled the accuracy of predicting which individuals would benefit from CBT compared with results from a conventional severity rating derived from questionnaires.
Another approach we used to assess the effectiveness of CBT combined two techniques. One, known as diffusion tensor imaging, evaluates how well connections established by fiber tracts, or white matter, enable different brain regions to communicate with one another. White matter consists of bundles of long, protruding extensions from neurons called axons that are covered in a whitish, fatty material known as myelin.
The second technique gauges what brain connections link together when a person lies at rest inside the MRI machine. With these data, researchers concocted a map of brain networks. From it, the team created a diagnostic measure, a biomarker, that produced a fivefold improvement in predicting which patients would benefit from CBT. Other studies, such as those by Greg Siegle of the University of Pittsburgh, have confirmed that a similar strategy seems effective in determining how patients with depression respond to CBT.
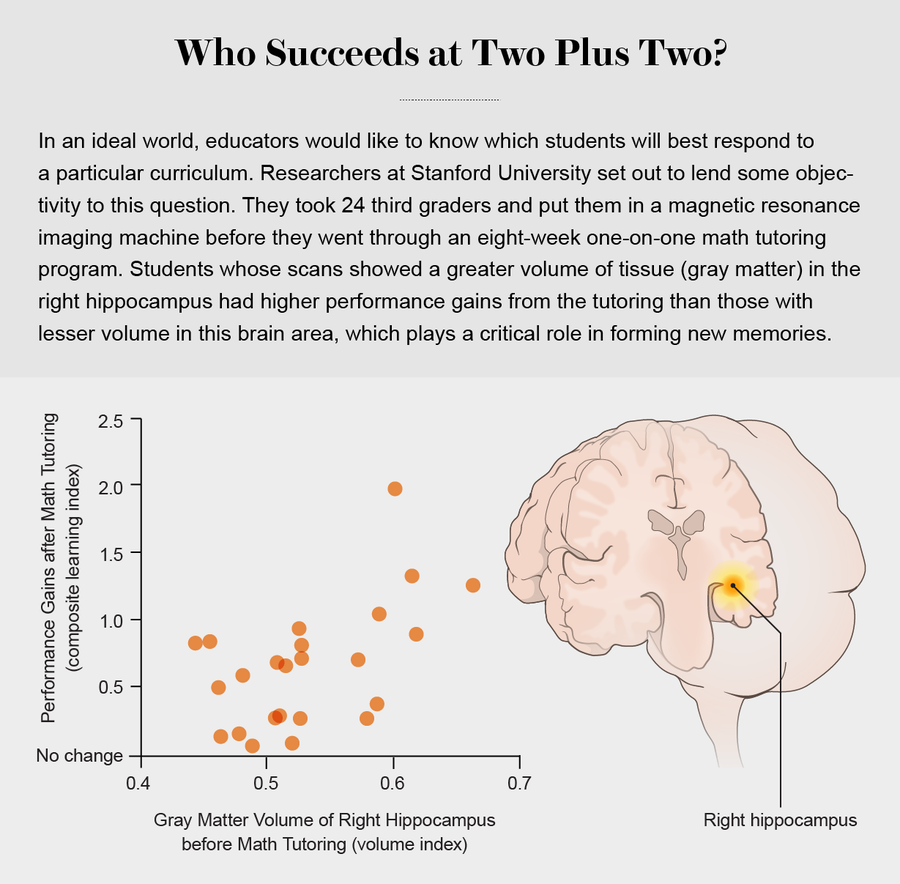
Credit: Falconieri Visuals (brain), Graphic by Jen Christiansen; Source: “Neural Predictors of Individual Differences in Response to Math Tutoring in Primary-Grade School Children,” by Kaustubh Supekar et al., in Proceedings of the National Academy of Sciences USA, Vol. 110, No. 20; May 14, 2013
Predicting the response to a drug for a psychiatric disorder can combine imaging with more conventional types of psychological tests. Andrea N. Goldstein-Piekarski of Stanford University and her colleagues examined responses to antidepressant medications. They interviewed patients about early life stress and then used fMRI to assess activity in the amygdala, a brain structure that processes emotions. In the scanner, patients looked at images of a series of happy facial expressions. Combining information about a person's early life stress and his or her amygdala's responses to faces hinted at whether that individual would benefit from antidepressant medications. The Siegle and Goldstein-Piekarski studies did not compare talk therapy with medication. But Helen Mayberg of Emory University has shown that brain imaging can also reveal whether an individual with depression is more likely to be helped by talk therapy versus a medication.
Predicting Relapse
Treatments for alcoholism, drug addiction, smoking and obesity share the aim of having users abstain or pare back their use of drugs, tobacco or food. Here, too, imaging techniques may play a role in predicting who will relapse into addictive habits. Half of patients treated for alcohol abuse go back to drinking within a year of treatment, and similar reversion rates occur for stimulants such as cocaine.
There is little scientific evidence for determining the length and duration of programs such as a 28-day in-patient rehabilitation at a treatment facility. Research has yet to show whether a shorter or longer course of therapy would prove more effective. Ideally, studies could ascertain if a given patient will relapse in six months or a year, allowing program length to be tailored to an individual's needs.
Imaging studies that make predictions of the outcome for alcohol and drug dependency and obesity are not as common as those looking at depression. Still, a number suggest that brain measures might foresee who will succeed in abstaining after treatment has ended. A study at the University of California, San Diego, found that brain imaging performed at the end of treatment for methamphetamine abuse predicted which patients would relapse during the following 12 months.
In an obesity-prevention study using MRI imaging at the University of Alabama, investigators discovered that reward areas of the brain that direct attention to food—the nucleus accumbens, the anterior cingulate and the insula—became active in a group of 25 obese and overweight individuals who looked at images of high-calorie fare before entering a 12-week weight-loss counseling program. Greater activation in these areas predicted who would have the most difficulty in shedding pounds once the program was over. Participants who went into the scanner afterward and who showed high activation of the insula and other attention and reward processing areas had more difficulty in sticking with the regimen nine months later.
Brain imaging may even help formulate the types of messages that health professionals use to encourage patients to adopt healthy behaviors. Emily Falk, then at the University of California, Los Angeles, and her colleagues asked those in their study to learn the proper technique for using sunscreen to prevent sunburn and skin cancers. Researchers recorded fMRI responses as participants watched slides that prescribed preventive measures. Participants then described their attitudes toward sunscreen use and their intentions to use it after receiving a supply of sun-protective towelettes. A week later the group received e-mails asking whether they had actually applied the lotion. Individuals who had logged greater activity during the viewing session in one brain area, the medial prefrontal cortex, which regulates beliefs and a sense of self, ended up using more sunscreen. Brain scans provided an objective measure of the program's effectiveness, extending beyond an individual's subjective evaluation of whether the health information helped.
Observation of brain activity may also assist in discovering the best approach to dissuade people from continuing to smoke. A 2010 paper in Biological Psychiatry from Harvard Medical School found that among 21 women, a high response to smoking-related pictures in two brain regions—the insula and the dorsal anterior cingulate cortex—forecast an inability to quit.
Better Learners
Children's education might benefit as well from brain imaging to predict difficulties in learning to read (dyslexia) or do math (dyscalculia). Teachers and parents try to help, but education operates largely on the model of waiting to fail. Students receive some guidance from teachers until they reach a point when they become discouraged, and then learning tends to break down.
What if instructional support did not merely react to failures but could anticipate specific forms of teaching that could be adapted to the needs of individual students? Some recent findings indicate that brain imaging can help predict students' future performance. Brain assessments, in fact, can sometimes outperform conventional educational and psychological measures at foreseeing how well a student will do in the classroom.
Among children with dyslexia, individuals vary greatly in their ability to compensate for reading difficulties by devising their own strategies that let them catch up to their classmates. Fumiko Hoeft, now at the University of California, San Francisco, and I measured brain fMRI responses to printed words in children with dyslexia around 14 years of age who also received extensive psychological testing. Then we examined the same children again 30 months later to see how much they might have improved in reading. About half the children exhibited substantial gains.
None of the standard educational testing measures correlated with future reading progress, but the brain scans combined with an analytical technique could make such predictions. Pattern-classification analysis, which delves into the complex data from fMRI brain scans using “big data” machine-learning algorithms, yielded more than 90 percent accuracy in characterizing whether a dyslexic child's reading would improve or continue to flounder two years after the images were captured. Other researchers have reported that electrical responses on the scalp (evoked response potentials) in young, preliterate children also predicted reading skills. Knowing what lies ahead may allow interventions prior to encountering reading difficulties, a strategy that spares children the sense of failure evoked by early struggles.
Math teaching may also profit. A study conducted by Vinod Menon of Stanford found that brain anatomy could identify whether a third grade student had more of a chance of benefiting from a math-tutoring program that encouraged students to shift from counting to arithmetic fact retrieval (memorizing 2 + 3 = 5, for instance) as a basis for arithmetic problem solving. Conventional behavioral tests of math abilities or IQ failed to predict which student would not be helped by the program, but brain measures succeeded. In particular, the size of the right hippocampus, an area associated with memory, correlated with how much a student would progress.
These studies hold promise of laying the basis for a neuroscience-based methodology of personalized learning. If this research can eventually identify the best instructional approach for a student, educators could avoid the failures that occur later in childhood or adolescence when learning difficulties become more difficult to correct.
Wanted: Better Predictions
If brain measures show such promise for predicting whether an individual will respond to a mental health treatment or schooling, why are these methods not already in use? Several challenges linger before these techniques enter clinics and schools. First, the predictions need additional statistical rigor. In the studies so far, models have linked brain activity to already known outcomes, such as how much an individual benefited from a treatment. In that sense, they might be called postdiction rather than prediction. New studies must now ascertain whether these findings routinely make accurate forecasts.
For the prediction sciences to move forward in mental health and education, the research community must begin to design studies that compare results for two independent groups. A mathematical model from one group can be tested on the other to validate the model.
One intriguing approach known as leave-one-out cross-validation excludes an individual from the overall evaluation of the results of the group under analysis. Researchers create a model from other individuals in the study to predict a particular health or educational outcome. The model then goes on to forecast a result for the left-out individual. The entire process repeats for each study participant with the goal of creating a model that better guides selection of each new patient's treatment. Only a handful of studies have achieved such a high standard to date, but this level of rigor must be met for the practical use of brain imaging as a prediction technique.
Another barrier relates to the cost and availability of MRI brain imaging. Any economic calculation must balance the price for the procedure, which is often about $500 to $1,000 per hour, against the prospect of having to pay for physician and hospital visits, lost work productivity and special education resources to support students falling behind. In some cases, other technologies might substitute for MRI, even while borrowing knowledge gleaned from the more expensive technique. Electroencephalography, which measures the brain's electrical activity, might, for instance, be adapted to take the place of MRI in some types of testing.
The promise and potential controversy surrounding MRI for clinical use show up in two recent studies. One by Joseph Piven of the University of North Carolina at Chapel Hill used fMRI to image 59 infants who were six months old to detect a heightened risk for autism spectrum disorder (ASD). The defining social and communication difficulties of autism rarely emerge at birth but typically only, with careful evaluation, at two years of age. Imaging studies of brain network activity at six months predicted correctly nine of the 11 infants who would be diagnosed with ASD some 18 months later. And the measurements also established that the other 48 would not be so classified. This kind of prediction could one day both calm the worries of parents whose infants will not progress to ASD and assist in devising early interventions to aid children at high risk.
Another prediction study attempted to build on evidence that impulsivity appears as a major risk factor for recidivism. A measure of brain activity for self-control could potentially help address the limited accuracy of expert advice in making decisions about bail, sentencing and parole. Kent Kiehl, a professor of psychology, neuroscience and law at the University of New Mexico, examined brain activity during an impulse-control task in 96 male offenders before their release from prison and then followed these men over a period of several years. The offenders performed a task during brain imaging intended to make impulse control difficult. They had to press a button as the character “X” appeared repeatedly on a computer screen. At the same time, they had to resist the temptation to press the button in the rare instances that the letter “K” appeared, thereby creating two conflicting impulses, depending on what was displayed on the screen.
The lab task helped to predict what happened to the men after their release from prison. The likelihood that a former inmate would face another arrest over a four-year period doubled if the offender had diminished activity in the anterior cingulate cortex, a brain region involved with cognitive control and resolving conflicting impulses. Brain scanning helped to better forecast future rearrest than conventional measures alone, such as scores on a psychopathy checklist, age or lifetime substance abuse. An unpublished reanalysis of the data by Russ Poldrack, now at Stanford, suggests that the strength of this prediction lessens considerably when applying these results to prison populations other than the one surveyed (a suggestion partially countered by Kiehl and his colleagues).
All these studies raise a set of critical issues. How accurate should a prediction be to improve mental health treatments and educational practices? As a corollary, how can predictions made from brain scans help people rather than curtail their educational or employment opportunities? If we could better project future mental health or learning difficulties or even criminal activities, how would we, as a society, ensure that such predictions do not justify punitive policies and instead promote individual well-being? Ironically, the better prediction becomes over time, the more pressing the need emerges for an ethical framework to use such knowledge wisely.