As astronomers gaze into the depths of space, they do so with unease: They don’t know precisely what the universe is made of.
It’s not just the true nature of dark matter that eludes them; so does the essence of the stars that speckle the sky and populate the many galaxies throughout the cosmos. Surprisingly, no one knows the stars’ exact chemical composition: how many carbon, nitrogen and oxygen atoms they have relative to hydrogen, the most common element.
These numbers are crucial, because they affect how stars live and die, what types of planets form and even how readily life might arise on other worlds.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Twenty years ago, astronomers expressed confidence in the numbers they had been working with. Now, not so much. The problem lies not in the far corners of the cosmos, but much closer to home. Astonishingly, scientists don’t know exactly what the sun is made of. As a result, they don’t know what the other stars are made of, either.

Although the sun’s exact oxygen abundance is controversial, no one disputes that stars much more massive than the sun — similar to the brightest stars now being born in the Orion Nebula (shown) — forged most of the oxygen found today on Earth and throughout the universe. Credit: NASA, ESA, M. Robberto (Space Telescope Science Institute/ESA) and the Hubble Space Telescope Orion Treasury Project Team
“The sun is a fundamental yardstick,” says Martin Asplund, an astrophysicist at the Max Planck Institute for Astrophysics in Garching, Germany. “When we determine the abundance of a certain element in a star or a galaxy or a gas cloud anywhere in the universe, we use the sun as a reference point.”
That makes sense. The sun constitutes 99.86 percent of the solar system’s mass. Any pollster who consulted the same percentage of voters would have no problem predicting the outcome of the next election.
The sun’s location in the Milky Way also makes it a good representative of the entire galaxy. Just as political opinions vary from the urban core to the countryside, so stellar abundances change from the galactic center to the edge, and the sun happens to be in the perfect position — about halfway from the Milky Way’s center to the edge of its disk of stars — to sample the whole galaxy.
What’s more, most stars in the universe reside in giant galaxies like the Milky Way, which makes the sun a touchstone for the entire cosmos.
Plus, the sun is so bright that astronomers can study details of its light with exquisite precision. That should allow them to determine the exact abundances of the sun’s chemical elements.
For nearly a century, astronomers have judged stars normal or not by seeing whether their chemical compositions match the sun’s. Most stars near us do; some don’t.
That’s why the article on the sun’s chemical composition by Asplund and his colleagues in the 2009 Annual Review of Astronomy and Astrophysics has garnered more than 4,000 academic citations from fellow scientists: Astronomers constantly compare stars and galaxies to the sun. It’s the universal standard.
But Asplund’s work is controversial. He and his colleagues have used new models to analyze sunlight and found drastically lower levels of the most common heavy elements in the sun — including carbon and oxygen — compared with previous calculations. (Astronomers call most elements heavier than helium “heavy.”) Asplund’s work therefore implies that the other stars and indeed the entire cosmos have a much smaller quantity of heavy elements than previously thought.
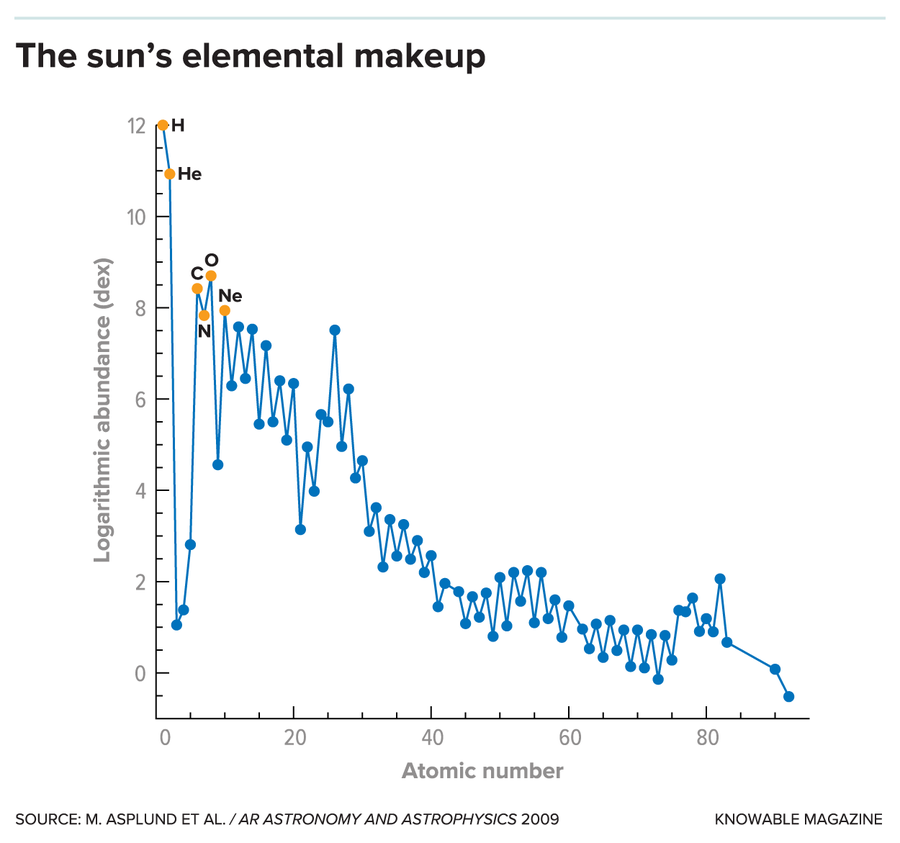
How much of the four most common heavy elements — oxygen, carbon, neon and nitrogen — does the sun contain? This chart shows relative abundances for dozens of elements (blue dots, most common elements labeled), expressed on a logarithmic scale where the number of hydrogen atoms is set at 12. (An element with an abundance of 11 is one-tenth as common as hydrogen; if 10, it is one-hundredth as common; and so on.) In 1989, the standard oxygen abundance was 8.93, which meant there were 1,175 hydrogen atoms for every oxygen atom. In 2009, however, Martin Asplund favored an oxygen abundance of only 8.69, which meant there were 2,042 hydrogen atoms for every oxygen atom. The estimated abundances of carbon, nitrogen and neon also plunged.
Take oxygen. “This is the most abundant heavy element in the universe,” says Marc Pinsonneault, an astronomer at Ohio State University. He has been a critic of Asplund’s numbers because they lead to conflicts with observations of the sun’s interior.
“The sun is one of the only ways we have of actually measuring how much oxygen there is. So if Asplund is correct … that means there’s 40 percent less oxygen in the universe period, because all of our measurements get multiplied by whatever we assume for the sun,” Pinsonneault says.
The controversy has endured for 20 years; neither side has yielded to the other. “We haven’t found the answer yet,” says Katharina Lodders, a cosmochemist at Washington University in St. Louis who divines abundances from meteorites and calls the long-standing dispute frustrating. “I think the ‘What are we missing?’ is one of the biggest challenges for scientists. How can that be, that there is something we cannot explain? There must be an answer.”
The lower levels of oxygen and other heavy elements that Asplund advocates have caused not just uncertainty but also trouble. “I suspected very early on that it would lead to a conflict,” he says.
Yet both Asplund and Pinsonneault say that the debate is a friendly one. “We disagree very strongly on the scientific interpretation,” Asplund says, “but we are very happy to go out for a beer afterwards.”
Fortunately, a variety of current and future experiments may finally resolve the matter.
Oxygen: A critical element
Despite the controversy, everyone agrees on the basics: The sun consists mainly of hydrogen and helium, the two lightest elements. It generates energy at its center through nuclear reactions that convert hydrogen into helium. But because of Asplund’s work, the amounts of the next most abundant elements are all in dispute.
It matters hugely. Oxygen accounts for nearly half of all heavy atoms in the universe. Most of these atoms trace their birth to stars much more massive than the sun. Late in their bright but brief lives, these stars fuse four helium nuclei together to make oxygen. The stars eventually explode, shooting the life-giving element away. Just one supernova can eject more than a solar mass of oxygen. If the oxygen level in the sun and thus the whole universe is as low as Asplund believes, these massive oxygen-producing stars have been much less prolific than has been thought.
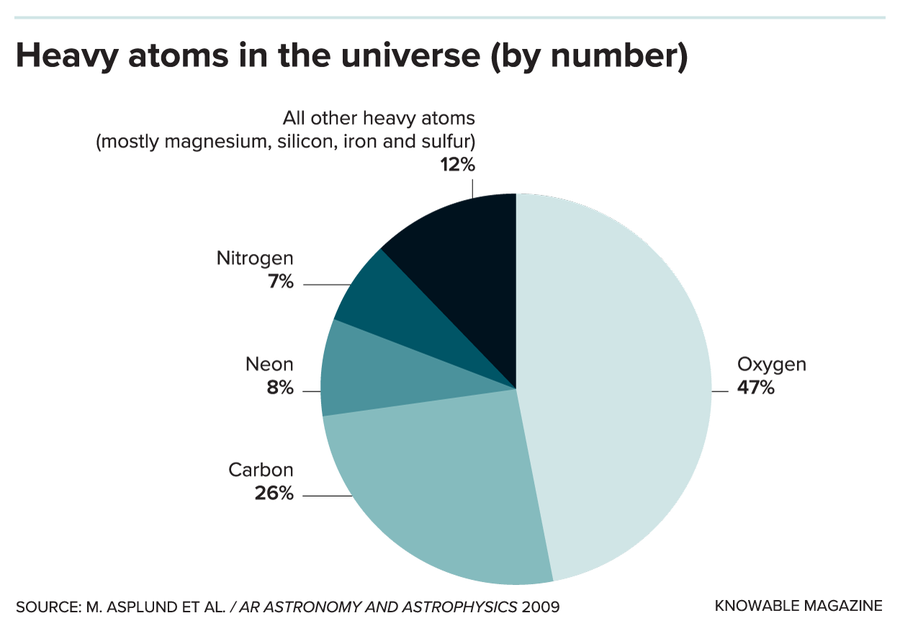
Nearly half of all heavy atoms in the universe are oxygen (as measured by number of atoms, not by weight). And just four elements — oxygen, carbon, neon and nitrogen — account for 88 percent of all heavy atoms, but their exact number relative to hydrogen has been in dispute.
Oxygen is vital in ways both obvious and not. The obvious: We need oxygen to breathe. The less obvious: More than half of the atoms in the rocks beneath our feet are oxygen. And the element played an important role in the formation of all the planets in our solar system.
Oxygen’s critical importance doesn’t end there. After all, there’s an oxygen atom in every water molecule. “Water is essential for life,” says Lodders. “Water was essential for forming life.” So no oxygen, no water and no life.
Carry on, wayward sun
Far-reaching though it is, the simmering controversy over the sun’s abundance of oxygen and other heavy elements started by accident. In the late 1990s, Asplund wanted to study ancient stars that had only a pittance of heavy elements. First, though, he thought it wise to better ascertain the sun’s composition.
To do so, he and his colleagues developed new models to explain the solar spectrum, the rainbow of colors our star gives off. Atoms of different elements absorb different wavelengths of light, producing what are called spectral lines. The more atoms of a particular element that exist on the sun’s surface, the more light the atoms absorb and the stronger the spectral lines. Spectral lines thereby can reveal an element’s abundance relative to hydrogen, which is the sun’s main ingredient.
Because the sun sets the standard, scientists can metaphorically see the entire universe in a single sunbeam: By analyzing the solar spectrum, they can determine the proportions of hydrogen, carbon, nitrogen and oxygen throughout the whole cosmos.
Asplund’s new models were much more sophisticated than previous ones, eschewing simplifications and approximations. “I had no real expectation that this would change the solar abundances at all,” he says. “It was kind of a lucky shot.”
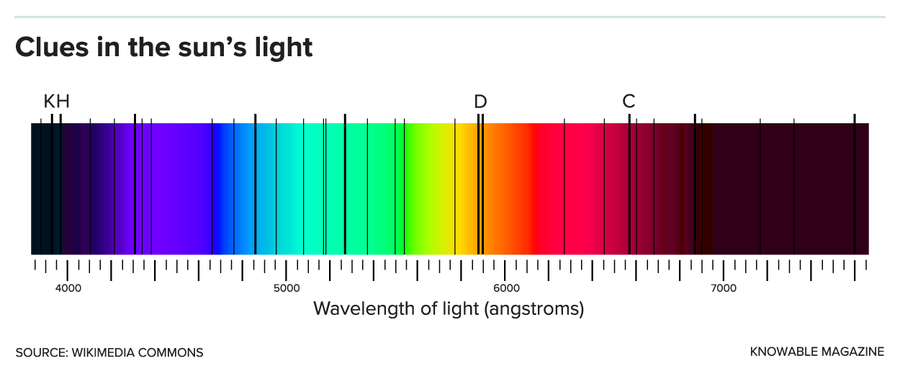
The solar spectrum (shown) can be analyzed to reveal clues to the sun’s makeup. Atoms on the sun’s surface absorb specific colors, leaving dark spectral lines in the observed spectrum. Each line’s strength tells of an elemental abundance. The H and K lines in the deep purple arise from calcium; the pair of yellow-orange D lines from sodium; and the red C line from hydrogen. Oxygen’s spectral lines are difficult to analyze.
In his models, each of the universe’s four most abundant heavy elements took a major hit. Compared with numbers published 20 years earlier, the 2009 article by Asplund and colleagues recommended sharply lower values. The new models slashed the estimated oxygen level in the sun and thus in the universe by a whopping 42 percent. Carbon, another prerequisite for life, fell 26 percent, while the neon and nitrogen levels plummeted 31 percent and 40 percent, respectively.
By all calculations, these four elements account for the vast majority (88 percent in Asplund’s work, a bit more in other numbers) of all heavy atoms in the universe. If Asplund was right, the universe had far fewer of them than anyone had thought. And that meant huge trouble for models of the sun’s interior.
Inside the sun
Heavy elements such as oxygen alter the sun’s interior, because they absorb radiation as it wends its way outward from the solar core to the surface. Using the old solar abundances, astronomers thought they had the sun’s interior figured out, thanks to a technique known as helioseismology. Just as our world has earthquakes, so the sun’s interior vibrates with sound waves. And just as seismologists use quakes to deduce the structure of the Earth’s interior, so the vibrations rippling through the sun have revealed its inner structure.
For example, in most of the sun’s interior, radiation bounces from atom to atom, slowly carrying heat from the core outward. In the outermost parts of the sun, however, material is cooler and more opaque, largely because heavy elements, such as oxygen, absorb photons. This opacity means photons can’t ferry heat there. Instead, a process called convection sets in: Hot gas rises to the solar surface, radiates heat, then cools and sinks back down. You see something similar when you boil a pot of water.
Helioseismology pinpoints the position of the boundary between the sun’s radiative interior and its convective envelope. “That shows up as a glitch in the sound waves,” Pinsonneault says. As a result, we know that this boundary occurs at precisely 71.3 percent of the solar radius. But if the sun actually has less oxygen, carbon, neon and nitrogen, then the sun’s interior is less opaque, allowing radiation to carry heat farther from the sun’s center, contradicting the helioseismological observations. “Either we don’t understand the sun or the [new solar abundances] are wrong,” Pinsonneault said at a 2011 talk where he favored a higher oxygen abundance.
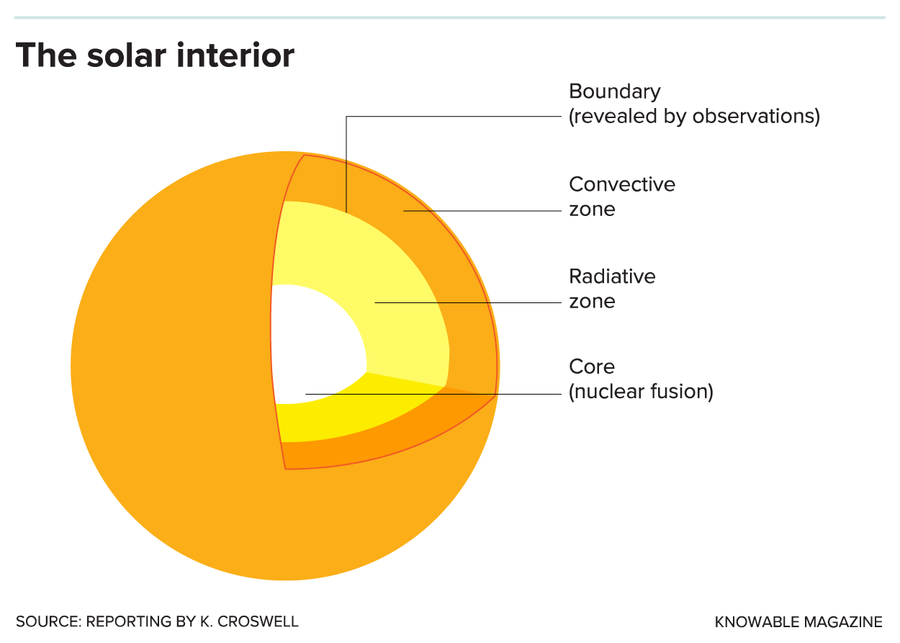
Nuclear reactions in the sun’s core produce energy, which is then transported outward by radiation and then by convection. The position of the boundary between the radiative zone and the convective zone has been revealed by helioseismological observations. The old elemental solar abundances put this boundary at exactly the observed position; the revised elemental abundances do not.
Still, Pinsonneault acknowledges that Asplund’s new models are superior to earlier ones and their redetermination of the solar abundances should be valid. For one thing, Asplund’s models take into account convection, which earlier work had neglected. His team also recognized that a red spectral line which supposedly arose from oxygen is actually a blend of oxygen and nickel; subtracting nickel’s contribution led to a lower oxygen abundance.
Much of the problem stems from the oxygen atom itself. “It’s just a trouble child,” Pinsonneault says. “It’s always been a trouble child.”
Common though oxygen is, it produces few spectral lines in sunlight, all of which are hard to analyze, so the element leaves few clues to its abundance. “By contrast, everybody agrees on the solar iron abundance,” Pinsonneault says. That’s because iron produces a plethora of spectral lines that are ripe for analysis.
Like Lodders, Pinsonneault calls the seemingly eternal dispute frustrating. “It’s been surprisingly hard to get new information to resolve the problem,” he says. “We just need new data to be able to crack this.”
Something new under the sun
Fortunately, fresh data will be coming soon. In the laboratory, physicists can measure the opacities of different elements by subjecting them to the torrid temperatures that prevail inside the sun. In recent years, scientists have coaxed these experiments to even higher temperatures — hot enough to probe conditions similar to those deep beneath the solar surface, at the convective-radiative boundary — and in plasmas sufficiently large and long-lived to yield accurate numbers.
In 2015 Jim Bailey, an experimental physicist at Sandia National Laboratories, and his colleagues reported that the opacity of iron in the sun is indeed higher than expected. “Our result made the astronomy community pretty happy,” he says, “because it means that there’s at least a hope that they can reconcile what they think are the best abundance estimates with the standard solar model and with helioseismology.”
Bailey has now turned his attention to oxygen and expects his first results in three years. If oxygen proves to be more opaque than currently calculated, then the sun doesn’t need as much of the element to maintain the observed location of the radiative-convective boundary. That could eliminate the discrepancy between the new solar abundances and helioseismology.
Meanwhile, both Asplund and Pinsonneault point to another promising solution. As the sun’s core generates energy, it emits neutrinos, ghostly particles that zip away and reach the Earth about eight minutes later. Ongoing studies of these neutrinos should offer a new way to estimate elemental abundances. That’s because certain neutrinos arise in a process that uses carbon, nitrogen and oxygen as catalysts to convert hydrogen into helium.
This CNO cycle generates only about 1 percent of the sun’s energy, but the more carbon, nitrogen and oxygen the sun truly has, the more of these CNO neutrinos should exist. Six years ago, physicists used the Borexino experiment in Italy to detect neutrinos from the sun's main nuclear reaction. This week, the Borexino researchers announced that this same experiment has picked up the CNO neutrinos, meaning it's only a matter of time until they help unveil the solar abundances.
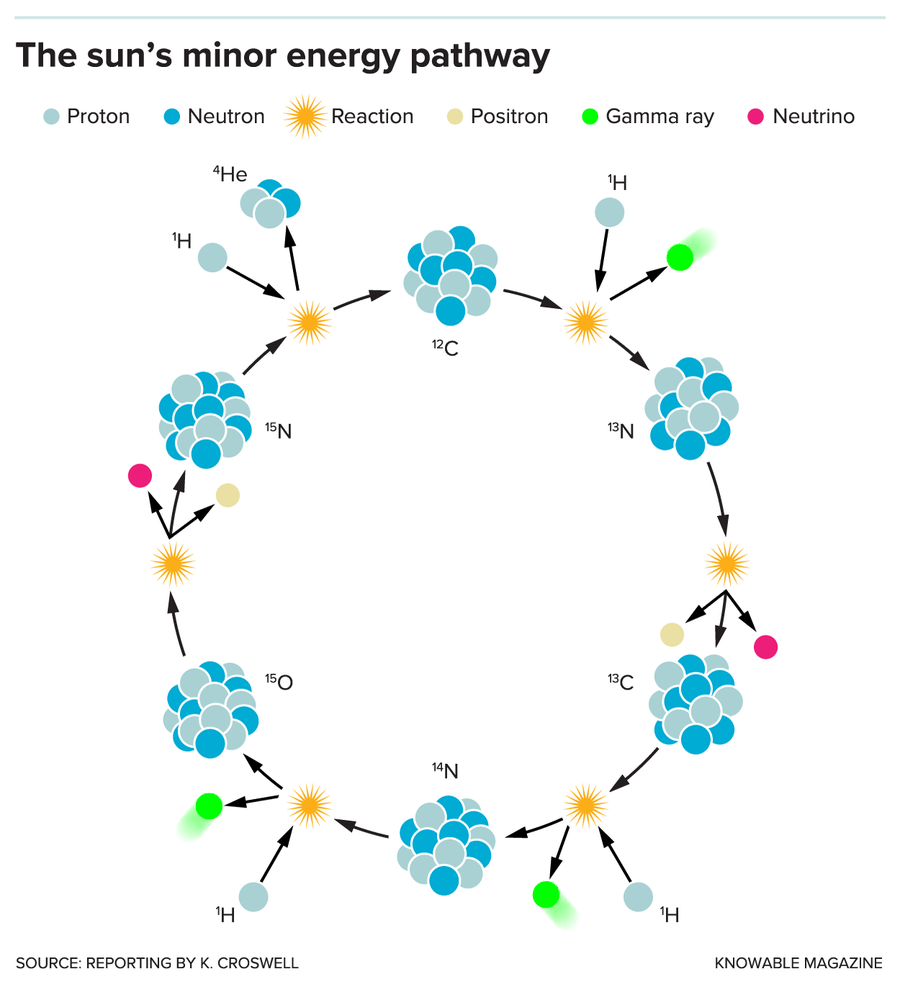
The CNO cycle generates only 1 percent of the sun’s energy but may someday reveal how much carbon, nitrogen and oxygen the sun contains. In this complex cycle, carbon, nitrogen, and oxygen nuclei catalyze the hydrogen-to-helium nuclear reaction but do not get used up in the process. The CNO cycle transforms four protons into one helium nucleus, creating energy and emitting two neutrinos (magenta). Physicists recently announced that they had been able to detect this type of neutrino for the first time.
The final verdict?
Lodders notes another reason for hope. Once upon a time, astronomers argued over the cosmic iron abundance: The solar spectrum gave a different level than meteorites did. “It was a big mystery for a long time,” she says. The debate ended when astronomers used newly measured atomic parameters for iron and revised their calculations of the solar iron abundance, vindicating the meteoritic result.
Asplund expects the ongoing opacity and neutrino experiments to resolve the controversy. “I wouldn’t bet my house on it,” he says, “but I would be very disappointed if we don’t actually know what the answer is in 10 years’ time.”
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews. Sign up for the newsletter.