When doctors ask Sara Gehrig to describe her pain, she often says it is indescribable. Stabbing, burning, aching—those words frequently fail to depict sensations that have persisted for so long they are now a part of her, like her bones and skin. “My pain is like an extra limb that comes along with me every day.”
Gehrig, a former yoga instructor and personal trainer who lives in Wisconsin, is in her mid-40s. At the age of 17 she discovered she had spinal stenosis, a narrowing of the spinal cord that puts pressure on the nerves there. She experienced bursts of excruciating pain in her back and buttocks and running down her legs. That pain has spread over the years, despite attempts to fend it off with physical therapy, anti-inflammatory injections and multiple surgeries. Over-the-counter medications such as ibuprofen (Advil) provide little relief. And she is allergic to the most potent painkillers—prescription opioids—which can induce violent vomiting.
Today her agony typically hovers at a 7 out of 10 on the standard numerical scale used to rate pain, where 0 is no pain and 10 is the most severe imaginable. Occasionally her pain flares to a 9 or 10. At one point, before her doctor convinced her to take antidepressants, Gehrig struggled with thoughts of suicide. “For many with chronic pain, it’s always in their back pocket,” she says. “It’s not that we want to die. We want the pain to go away.”
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Gehrig says she would be willing to try another type of painkiller, but only if she knew it was safe. She keeps up with the latest research, so she was interested to hear in 2024 that Vertex Pharmaceuticals was testing a new drug that works differently than opioids and other pain medications.
That drug, a pill called VX-548, blocks pain signals before they can reach the brain. It gums up sodium channels in peripheral nerve cells, and obstructed channels make it hard for those cells to transmit pain sensations. Because the drug acts only on the peripheral nerves, it does not carry the potential for addiction associated with opioids—oxycodone (OxyContin) and similar drugs exert their effects on the brain and spinal cord and thus can trigger the brain’s reward centers and an addiction cycle.
A young Pakistani firewalker had a genetic mutation affecting pain-signaling neurons, letting the boy walk on burning coals without feeling pain.
In January 2024 Vertex announced promising results of clinical trials of VX-548, which it is calling suzetrigine, showing that it dampened acute pain levels by about one half on that 0-to-10 scale. The company applied for U.S. Food and Drug Administration approval for the drug that same year.
Other pain drugs that target sodium channels are now being developed, some by firms motivated by Vertex’s success. Navega Therapeutics, led by biomedical engineer Ana Moreno, is even using molecular-editing tools such as CRISPR to suppress genes involved in chronic pain. “We are definitely hopeful that we can replace opioids, and that’s the goal here,” she says.
One in five U.S. adults—51.6 million people as of 2021—is living with chronic pain. New cases arise more often than other common conditions, such as diabetes, depression and high blood pressure. Yet pain treatments have not kept pace with the need. There are over-the-counter pills such as aspirin, acetaminophen (Tylenol) and nonsteroidal anti-inflammatories (NSAIDs) such as Advil. And there are opioids. The glaring inadequacy of existing medications to alleviate human suffering has fueled the ongoing opioid epidemic, which has led to more than 730,000 overdose deaths since its start.
VX-548 does have limits. It left some patients in significant discomfort, and so far it has been tested mostly in those with acute pain, not the much larger problem of chronic pain. Gehrig says she wants more assurances that the drug won’t cause nasty side effects before she takes it.
But the compound has shown that a new mechanism of pain relief is possible, says Stephen Waxman, a neurologist at Yale University who studies pain signals—and who is not involved in the Vertex clinical trials. Future drugs using that mechanism are likely to be even more effective, he notes. Waxman used to tell patients that a new means of managing their pain was on the way but that it may not happen for many years. “Now I can relax the caveat and say I think things are going to happen fairly quickly,” he says.
The pain medications that exist today are, in large part, derivatives of natural products that have been around for thousands of years. Aspirin originally came from willow bark. Morphine and codeine were derived from the opium poppy plant. Prescriptions for what evolved into the two major classes of pain drugs—NSAIDs and opioids—were etched on clay tablets by ancient Sumerians 4,000 years ago.
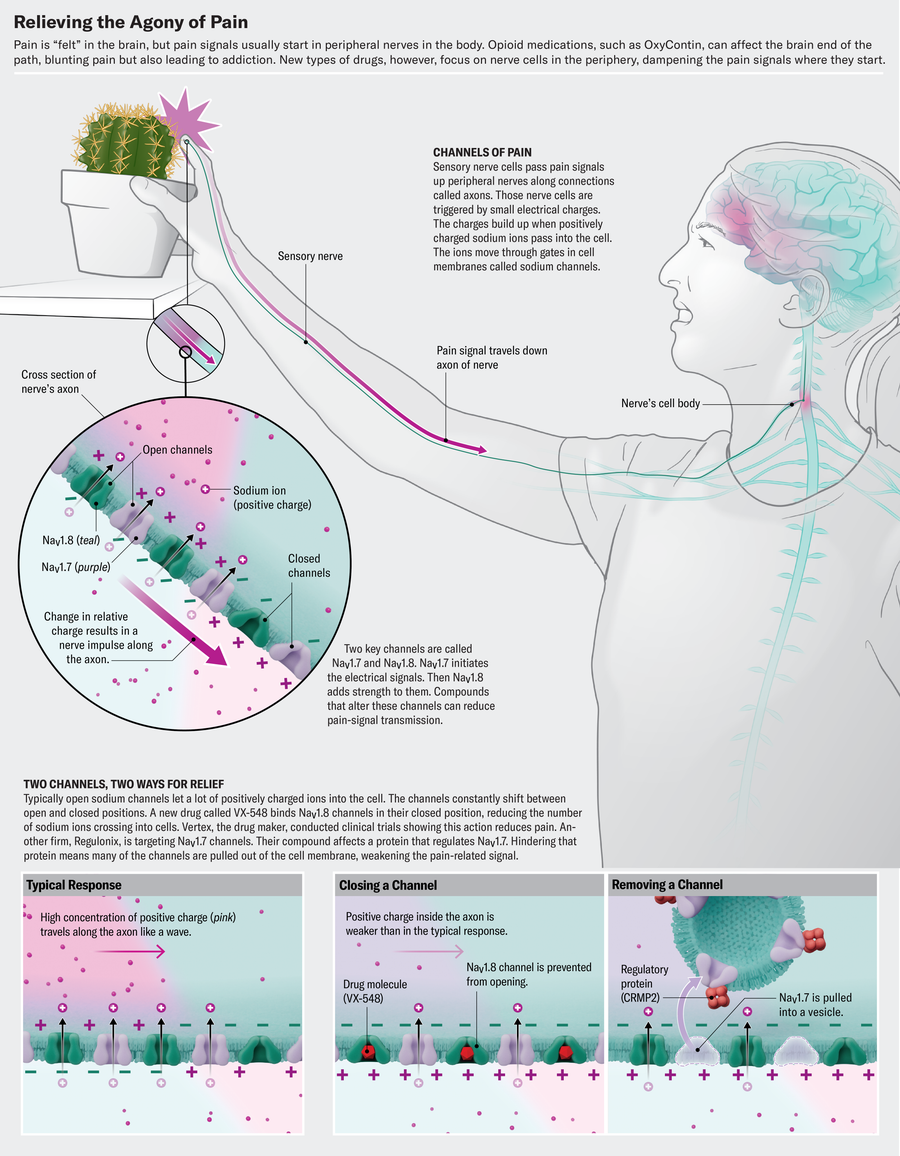
Modern research on the molecular mechanisms underlying pain, conducted during the past two decades, makes a different approach possible. Scientists know that our body is home to large numbers of pain-signaling nerve cells that innervate our skin, muscle and visceral tissues. These cells act like an alarm system, detecting threatening stimuli such as extreme temperatures, sharp objects or noxious chemicals. In response to these cues, they create impulses that carry pain signals along nerve fibers to clusters of cells known as dorsal root ganglia, which are tucked beside the spinal cord. From there, the signals continue their journey upward to the brain, where pain becomes reality. “This is the axis of pain,” says Rajesh Khanna, a pharmacologist and pain researcher at the University of Florida.
Central to this pathway are sodium channels, cellular gates scattered throughout the membranes of nerve cells. Whenever there is a shift in membrane potential, these gates open to allow the influx of sodium ions that generate the electric currents responsible for nerve impulses. Normally those pain signals serve a protective purpose—alerting someone to pull their hand away from a hot stove or noting inflammation or injury that needs to be addressed. But in chronic pain, those protective mechanisms can go awry.
A voltage-gated sodium channel (or NaV, Na standing for sodium and V for voltage) seems like the ideal target for treating pain; after all, if you can stop it, you can stop pain signals from being transmitted. Yet because these channels control electrical impulses that power the heart and brain, blocking them willy-nilly would impair vital functions. That’s why novocaine and lidocaine—which are sodium channel blockers—are used as local numbing agents but can cause serious side effects if administered systemically. So scientists trying to block these pain pathways searched for channels that act more often in the peripheral sensory nerves, eventually identifying three: NaV1.7, NaV1.8 and NaV1.9.
NaV1.7 and NaV1.8 are the pivotal players in pain signaling. “They work in tandem, like dominoes,” Waxman says. “NaV1.7 initiates the electrical signal, and NaV1.8 takes off, producing 80 percent of the current underlying the action potential.” (NaV1.9 plays a more niche role in setting the pain-signaling neurons’ threshold potential.)
Beginning about 20 years ago, a series of reports linked these channels to pain disorders in humans. A mutation in the SCN9A gene, which encodes NaV1.7, was discovered in a family in China who suffered from a rare condition called erythromelalgia, or “man on fire” syndrome. In people with this condition, mild warmth can trigger attacks of searing pain that feels like a blowtorch. Waxman found that mutations in patients with erythromelalgia made the NaV1.7 channel overactive, causing pain-signaling neurons “to scream when they should be whispering.” Elsewhere, researchers found a mutation with the opposite effect in a young Pakistani firewalker. That mutation extinguished the flow of pain-signaling ions through the NaV1.7 channel. As a result, the boy could walk on burning coals without feeling pain.
The discovery of the genetic basis of his condition—known as congenital insensitivity to pain—set off a race in the pharmaceutical industry to identify molecules that could block NaV1.7. The goal was to provide a similar pain-free existence to the rest of the population. “This was the holy grail. You have a protein, you mutate it, you have no pain—it’s got to be the target,” Khanna says. “A lot of pharma companies put a lot of money into this effort, but none of those compounds have been successful.”
Many compounds targeting NaV1.7 looked promising in the laboratory, only to fail in clinical trials. Pharma companies AstraZeneca and Genentech both developed candidates that stalled after phase 1 trials. Pfizer’s PF-05089771 failed to perform in a battery of tests evoking pain in healthy volunteers. Biogen scrapped development of its NaV1.7 inhibitor, vixotrigine, after lackluster results from a string of phase 2 trials in several types of neuropathic pain. After more than a decade of false starts, investment dwindled, and drug candidates disappeared from development pipelines.
“For many with chronic pain, it’s always in their back pocket. It’s not that we want to die. We want the pain to go away.” —Sara Gehrig, pain patient
In 2017 the White House declared a public health emergency for the opioid crisis, which was killing 91 people every day. That same year Francis Collins, then director of the National Institutes of Health, gathered industry leaders as well as basic scientists and clinicians to discuss strategies to combat the crisis. Sean Harper, who led R&D at biopharmaceutical giant Amgen at the time, remembers the meeting had representatives from about 20 of the world’s top pharma companies, and Collins asked what they had in the works. “It was sad,” Harper recalls. “There were very few companies that were working on anything other than tamper-proof, crush-proof opioid pills.”
Across the industry, novel pain-drug research stagnated. Amgen, which had identified a number of potential NaV1.7 inhibitors, eventually shuttered not only its pain research but also the bulk of its neuroscience program. In general, “I think what happened is people sort of felt that it was just too hard,” Harper says.
One big reason for the difficulty had to do with the nature of the targets themselves. The NaV channel family contains nine closely related members that share more than 50 percent of their genetic sequence. Because of this similarity, the sodium channel inhibitors developed in the 2000s were often unable to target one subtype without hitting others. “The selectivity was terrible, frankly,” says John Mulcahy, a chemist and CEO of the San Francisco based biotech firm SiteOne Therapeutics. “It’s taken a long time to overcome that.”
At Vertex, researchers believed that the compounds that had been tested before were simply not selective enough or didn’t attach to a channel for enough time and that to find molecules that worked they just needed to keep searching. To speed up their hunt, they had been working on a technology that could measure the effect of massive numbers of molecules, at various concentrations, on the opening and closing of several types of sodium channels. Traditionally, researchers have studied sodium channels using a laborious method called patch-clamp electrophysiology. The technique involves isolating part of a cell’s membrane, applying voltage to trigger its channels to open, adding one single potential drug, and then recording the oscillating waves of electrical activity.
In the early 2000s Vertex scientists Jesús González and Michael Maher designed a system called E-VIPR (for electrical stimulation voltage ion probe reader) to test many compounds against one channel very quickly. The system uses a high-density array of cells, each of which expresses one type of sodium channel. Tiny electrodes generate an electrical field that can stimulate the channels as many as 100 times per second. As these channels open, a voltage-sensitive dye shifts from orange to blue, and the color change is captured by a sophisticated optical detection tool.
Falconieri Visuals
“It’s a very quick process, faster than the human eye can detect, but incredibly rich in information,” says Paul Negulescu, Vertex’s senior vice president of research and head of its pain program. The engineering group developed the first generation of the technology about two decades ago, and it is on the third generation now. “That’s been the workhorse. And we have tested tens of thousands of compounds on the system that runs every day,” he adds.
With this method Vertex could extensively test how a potential drug interacts with a particular channel. Sodium channels undergo big shape changes as they open and close, with pieces of the protein moving up and down with dizzying speed. “It’s kind of like a bucking bronco,” Negulescu says. “The drug has to get on the bucking bronco and stay on while it’s going through its paces and eventually settle that bucking bronco down so it stops moving.” A drug candidate might land on the channel for a time, only to get kicked off once its gyrations prove too much. Or it might hop onto another channel, generating unwanted off-target effects.
Negulescu says that Vertex’s approach tries to mimic the physiological states of the sodium channel, putting it through multiple cycles of opening and closing to make sure any promising new drug stays put. “Most of the methods don’t do it that way,” he says. “And because of that, I believe we end up with pharmacology that isn’t translating when we get to people.” The company used its proprietary method to generate data on a variety of different sodium channels. As the industry continued to focus on NaV1.7, Vertex started to see success with NaV1.8 and pushed forward a program on the neglected channel. “I think we zigged when others zagged,” Negulescu says.
Vertex launched its first clinical trials of a NaV1.8 inhibitor in 2015. It wasn’t effective enough, and neither were the two immediately following it. But finally one was tolerated well by a small group of patients and relieved some of their pain. That was VX-548, and it prompted the company to move ahead with bigger studies in 2022.
Two years later, in January 2024, Vertex announced positive results of two large, pivotal clinical trials. The researchers enrolled about 1,100 people, each of whom was undergoing bunion removal or tummy tuck surgery, operations commonly used to model acute pain. Study participants got a placebo, VX-548, or the drug combo of hydrocodone (an opioid) and acetaminophen, known as Vicodin.
When measuring pain relief on the 0-to-10 pain scale, the new drug performed just as well as Vicodin without the addiction risk. Both treatments reduced pain by about three points, from about a 7 to a 4. And in the people recovering from abdominal surgery, relief kicked in more quickly with VX-548 than it did for those who got Vicodin.
The drug provided less relief than Vicodin for bunionectomy patients when using a different pain scale. Still, those taking VX-548 reported fewer side effects—such as nausea, constipation, headache and dizziness—than those on the placebo, indicating the treatment was generally safe. (Untreated pain in the placebo group could increase side effects because it can elevate stress levels, upsetting digestion or triggering headaches.)
Studies suggest that even a 3-point drop in pain can have a meaningful impact on quality of life. Gehrig, the Wisconsin patient, remembers a time when her pain level registered at a 4, and she was able to work. After a botched surgery sent her pain skyrocketing, she was forced to go on disability.
If approved, VX-548 could help people such as Gehrig by offering relief that lands somewhere in between that provided by drugs such as acetaminophen, which are safe but limited in their power, and stronger opioids, which come with serious risks. It could provide relief to patients who are allergic to or who simply cannot tolerate the other drugs. Moreover, it could open up options for individuals who want to avoid the risks of drug dependency.
Vertex applied for FDA approval of the drug for cases of moderate-to-severe acute pain. Many experts agree that although it makes sense experimentally to go after acute pain first, the bigger need is providing relief to people whose daily life is disrupted by chronic pain. Vertex scientists think the drug will work for that type of agony because the mechanisms underpinning chronic and acute pain are similar. It reported positive results from a smaller efficacy and safety trial of VX-548 in diabetic peripheral neuropathy, a common type of chronic pain caused by nerve damage from high blood glucose, and plans to move forward with a phase 3 trial.
In addition, the company launched a separate study testing the drug in a form of chronic lower back pain known as lumbosacral radiculopathy. And Vertex researchers continue to use their drug-discovery platform to evolve compounds that are more potent and more selective. “We are all about serial innovation,” Negulescu says. The company already has a next-generation NaV1.8 inhibitor, VX-993, moving toward phase 2 clinical trials.
Others in the pain field have been watching Vertex closely and are excited by its results. “I think the great contribution that Vertex has made here generating the clinical data that they have with their program is to make people understand that, hey, this is not a hopeless thing,” Harper says. He, with other investors, recently launched a company called Latigo Biotherapeutics to develop sodium channel inhibitors.
Waxman says the Vertex findings were modest yet important—so important that he called VX-548 “a game changer,” not because it will change clinical practice on its own but because it will transform the research pipeline. “This is going to be like the development of the statin drugs,” he says. “The first statin drugs were, in retrospect, not very good. But they set the stage and really were the impetus, and the ones we have now are life-changing.”
Only a handful of companies are openly developing pain therapeutics going after NaV1.8 or NaV1.7, which remains a viable target. More may be working in “stealth” (Merck’s patent activity indicates it is dabbling in the field), and others most likely will join the effort, emboldened by Vertex’s progress. Some are already designing small molecules to block the sodium channels or nearby proteins; some are modifying natural toxins to disable the transmission of pain signals; still others are using gene therapy to turn down the signal at its source.
Latigo, Harper’s start-up, is the latest to emerge in this space. In February 2024 the California-based biotech launched with $135 million in funding and a NaV1.8 inhibitor, LTG-001, in phase 1 clinical trials. Early on, the company pursued both NaV1.7 and NaV1.8. But Harper says that when it saw that one of Vertex’s drug candidates had achieved positive results in both acute and chronic pain models, that “helped to push NaV1.8 to the front of the queue.” Now Latigo has a few other small molecules it is getting ready to test. Harper notes that typically when a company has taken an entirely new class of medicines into the clinic, as Vertex has, there are many others “nipping at their heels.”
Previously, Harper says, the historical lack of investment in pain medicine made for “pretty light competition.” According to an analysis by BIO, a biotech industry trade group, investment in pain and addiction drug development is remarkably low given the societal burden of these diseases. In 2021 pain and addiction companies raised $228 million in venture capital. That represented only 1.3 percent of total therapeutic venture funding in the U.S. In contrast, oncology companies brought in $9.7 billion, or 38.3 percent of the venture funding pie. What’s more, most industry pain programs have focused on different formulations of opioid drugs rather than riskier forays into new mechanisms.
Michael Oshinsky, director of the Office of Preclinical Pain Research at the National Institute of Neurological Disorders and Stroke, says a leading reason for pharma’s persistent focus on opioids and neglect of other research avenues is that opioids have been a safer bet. “There’s a 30 percent chance to have a clinical trial for an opioid making it to the market. That’s really crazy high for therapeutics development. And it’s about a 0.7 percent chance for something that doesn’t hit the opioid receptor,” he says.
Oshinsky co-chairs the NIH’s Helping to End Addiction Long-term (HEAL) initiative, which aims to accelerate research on new nonaddictive pain meds. “What we do is we try to de-risk targets,” he says. The program has been helping up-and-coming developers of sodium channel inhibitors such as Regulonix, SiteOne and Navega by validating their targets, optimizing their compounds or testing their approaches in preclinical models.
When measuring pain relief on a 0-to-10 pain scale, the new drug performed just as well as Vicodin but without the addiction risk.
University of Arizona spin-off Regulonix is sticking with NaV1.7 as a target but is going after it differently than its predecessors. Rather than blocking the sodium channel, the company is trying to remove NaV1.7 from the cell membrane. Without the channel there will be fewer sodium ions that can cross into the cell. University of Florida’s Khanna, who co-founded Regulonix and is chief scientific officer of the company, says an early version of its compound successfully affected NaV1.7 signaling in rat, mouse and pig models of acute and chronic pain. But, he admits, “we’re nowhere close to being in humans.”
A different approach is to take naturally occurring sodium channel blockers—such as the tetrodotoxin that makes puffer fish so lethal—and modify them to block channels predominantly found in pain-sensing neurons. SiteOne, started by Stanford University scientists, is following this game plan. In 2022 it began a collaboration with Vertex to develop its therapeutic candidates that target NaV1.7. The company has also secured additional NIH funding to work on a NaV1.8 inhibitor called STC-004. “In our experience, NaV1.7 inhibitors can act almost like an on-off switch for pain,” Mulcahy says. But a NaV1.8 drug “is a little bit different—it’s more like a dimmer switch.”
Finally, instead of manipulating existing pain channels, some researchers are trying to keep so many of them from forming by reducing the activity of genes that encode them. That type of gene therapy is being pursued by Moreno and her company Navega. They are working with a technology that Moreno developed during her doctoral research at the University of California, San Diego. There she used CRISPR and its older gene-editing counterpart, zinc finger proteins, to target genes that help to build NaV1.7; the result was suppression or even prevention of pain in rodents. Since launching Navega, she and her team have shown that the approach works for various kinds of pain—including neuropathic, chemotherapy-induced, inflammatory, visceral and arthritic—and they are quickly advancing toward first-in-human trials.
“Because we have long-lasting results, we’re going to focus on intractable pain,” Moreno says. Navega plans to test the gene therapy in that rare subset of patients with “man on fire” syndrome, who have known mutations causing their pain, before thinking about larger, more complicated clinical trials for chronic pain. “We get e-mails all the time from patients who are suffering from all over the world,” she says. “It’s very motivating.”
For Gehrig, the prospect of adding a new and effective type of pain reliever to her medicine cabinet has given her hope. But she has tried new things before, only to be brought down by debilitating side effects. Gehrig says she will wait to try VX-548 until her doctor can assure her it is safe. The trials showing few side effects are important, she says, but she’d prefer that the drug be in clinical use for a while before she takes it herself.
For now she relies on other ways to cope with her pain. She runs six support groups for the U.S. Pain Foundation, including one for the LGBTQ community and another for people based in Wisconsin. After years of trying everything else, she has experienced the most healing from a daily practice of reflection and prayer, mindfulness and meditation. “It’s a constant listening to my body every day, really trying to learn self-love and self-compassion—that’s been my medicine,” she says. Her self-healing practices keep her going. But she wouldn’t mind a little more help from the medical world.
If You Need Help
If you or someone you know is struggling of having thoughts of suicide, help is available. Call the 988 Suicide & Crisis Lifeline at 988 or use the online Lifeline Chat at 988lifeline.org/chat or contact the Crisis Text Line at texting TALK to 741741.

